Irish Medicines Board - PharmaTrust - Controlled Drugs Licensing Extranet
Technologies: IIS, .NET, ASP.NET, Oracle
Customer Profile
The objective of the Irish Medicines Board (IMB) is to ensure in so far as possible, consistent with current medical and scientific knowledge, the quality, safety and efficacy of medicines available in Ireland and to participate in systems designed to do that throughout the European Union. Before a medicinal product can be authorised for use, an application must be made to the Irish Medicines Board and this must contain all of the necessary data supporting its quality, safety and efficacy.
The Irish Medicines Board carries out the following services within Ireland:
- Licensing of medicinal products for human use
- Licensing of veterinary products
- Licensing of wholesalers of human medicines
- Licensing of manufacturers of human and veterinary medicines
- Pharmacovigilance & Drugs safety monitoring
- Clinical Trial Licensing
- Inspection of wholesale and manufacturing sites
Business Situation
When the Irish Medicines Board (IMB) approached Engine Solutions they were using a client-server based application provided by the United Nations for managing licence applications. This system provided no online functionality. The system allowed for the inputting of the licence information, but this could only be done by IMB staff. The IMB were manually processing in excess of 800 paper based import and export licences application requests for controlled drugs substances, each year. This paper based processing was not only time consuming and costly for the IMB, but it was also very time consuming and costly for each of the three hundred plus establishments who must annually apply for the licences. A software solution was called for that could automate and streamline the application process for all parties involved.
Solution
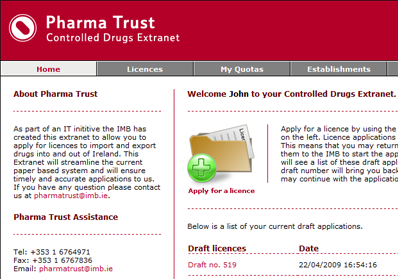
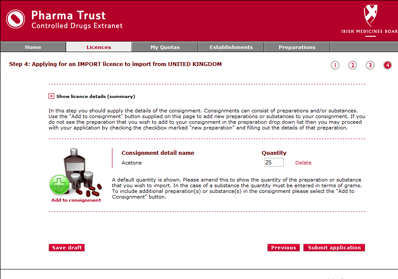
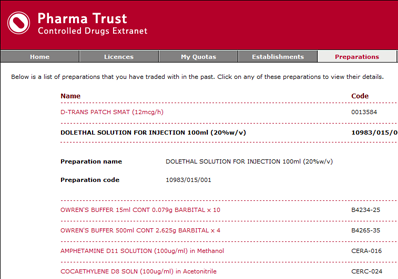
Engine Solutions suggested an online extranet application that each client establishment could log onto to submit their licence applications. The extranet automates as many elements of the application process as possible, such as suggesting common establishments that could be selected for trading with, and common controlled drugs substances that could be included on a licence application. The extranet provides shopping cart type functionality that allows establishments to build up the contents of their licence application before approving the overall licence application and submitting it to the IMB for approval.
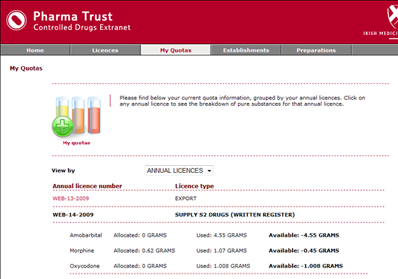
A second online application was developed for IMB’s Controlled Drugs department to allow them to manage the licence applications and the associated information. This application provides all of the administrative functionality necessary to manage the day-to-day running of the establishment’s extranet.
When licence applications are submitted through the online extranet, they are seamlessly created in the United Nations system. This means that the UN system could continue to be used for management and international reporting requirements.
Results
The return on investment for the Controlled Drugs project was almost immediate. Whereas the IMB originally had two people fully dedicated to processing controlled drugs applications, the application process is now completed automated. The new system also provides a greatly improved service to the IMB’s clients. Establishments now spend significantly less time on the application process and can now have licence applications approved in days rather than weeks.
Testimonial
"In all of the projects that Engine have undertaken for the Irish Medicines Board they have been professional and instrumental in the delivery and success of the projects. Throughout each of the projects they have demonstrated their ability to understand the business requirements and define technical solutions that meet those requirements, and have delivered solutions that are both scalable and useable. We have no hesitation in recommending them and will be continuing to work with them on current and future projects."
Kevin Horan | Director ICT & Business Services
Irish Medicines Board
www.IMB.ie