Health Products Regulatory Authority - Website, Content Management System & Mobile Apps
Technologies: IIS, .NET, ASP.NET, SQL Server, Sitefinity CMS, Web Services, Android, iOS
Customer Profile
The Irish Medicines Board (IMB) changed its name to the Health Products Regulatory Authority (HPRA) on 1 July 2014. The HPRA’s role is to protect and enhance public and animal health by regulating medicines, medical devices and other health products.
The HPRA’s remit and regulatory functions include:
- Human medicines
- Veterinary medicines
- Clinical trials
- Medical devices
- Controlled drugs
- Blood and blood components
- Tissues and cells
- Cosmetic products
- The protection of animals used for scientific purposes
- Organs intended for transplantation
Business Situation
The requirement for redevelopment of their previous website came about due to a number of factors, primarily their widening remit where the addition of new functional areas required an online presence. Recent EU directives on drug safety and falsified medicines included legal obligations to provide information online.
The HPRA required an enterprise content management system that would allow them to manage and publish content across the organisation. The new website would also need to integrate with several internal HPRA systems to publish medicine information on the website and allow the public and healthcare professionals to report adverse incidents to the HPRA.
The HPRA underwent a change in brand identity in 2014. A new logo, associated corporate identity and a new website were required to support these changes.
Solution
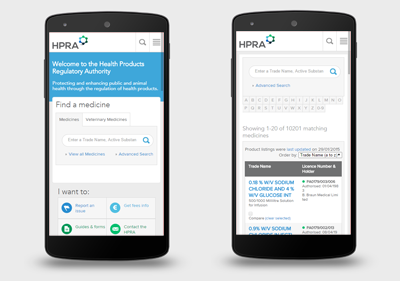
Engine conducted design, information architecture and requirements workshops with key HPRA staff. The results of these meetings fed into our analysis work to produce a detailed specification of the new website. An extensive poll was also conducted to get feedback from a broad range of website users. New responsive website designs were produced so that the new website would function across all devices.
The Telerik Sitefinity product was configured and enhanced to deliver an enterprise content management system that delivered on the requirements of the HPRA. This allowed the HPRA to centralise the workflow of their content creation and create a multilingual website.
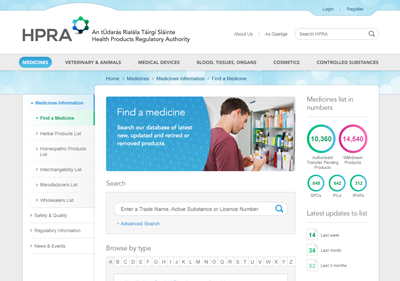
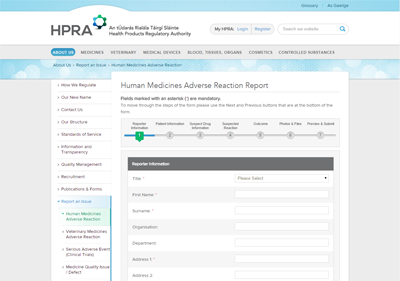
The new website was integrated with internal HPRA systems to publish all medicines and generics that are available in the Irish market as well as registries of all authorised manufacturers and wholesalers in Ireland. Registrations for internal systems are also processed through the website as well as adverse incident reports from the public and healthcare professionals.
Results
The new HPRA website is a multilingual, mobile and tablet friendly website that groups information and services based on three key stakeholder groups:
- Patients & Public
- Healthcare Professionals
- Industry
Up-to-date information on over 10,000 medicines is available online as well as over 150 lists of generic medicines making it the largest resource of information on medicines in Ireland. Website users can follow medicines and groups of medicines, and receive SMS and email alerts relating to them.
Patients, healthcare professionals and industry can report safety and quality concerns directly to the HPRA using online forms on the website and receive a reference number and full copy of their submission.
Centrally managed through an enterprise content management system, Engine Solutions enabled staff across all HPRA departments to publish content through a workflow approval process.
Testimonial
"In all of the projects that Engine have undertaken for the Health Products Regulatory Authority they have been professional and instrumental in the delivery and success of the projects. Throughout each of the projects they have demonstrated their ability to understand the business requirements and define technical solutions that meet those requirements, and have delivered solutions that are both scalable and useable. We have no hesitation in recommending them and will be continuing to work with them on current and future projects."
Kevin Horan | Director ICT & Business Services
Health Products Regulatory Authority
www.hpra.ie