Heads of Medicines Agencies - Central European Submission Platform
Technologies: IIS, .NET, ASP.NET, SQL Server, sFTP
Customer Profile
The Heads of Medicines Agencies is a network of the Heads of the National Competent Authorities whose organisations are responsible for the regulation of Medicinal Products for human and veterinary use in the European Economic Area. The Heads of Medicines Agencies is supported by working groups covering specific areas of responsibility and by the Heads of Medicines Agencies Management Group and Permanent Secretariat.
Business Situation
The Heads of Medicines Agencies (HMA) is currently undertaking a project aimed at establishing a single portal for electronic submissions to multiple competent authorities in the context of European registration and post-approval procedures in the European Economic Area.
CESP is an initiative of a group of EU Member States and industry representatives, managed by HMA, and is available for all EU Member States and authorisation applicants and holders, for both human and veterinary medicinal products. Current participants include agencies from Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Iceland, Ireland, Italy, Malta, The Netherlands, Norway, Portugal, Slovenia, Spain, Sweden and the UK.
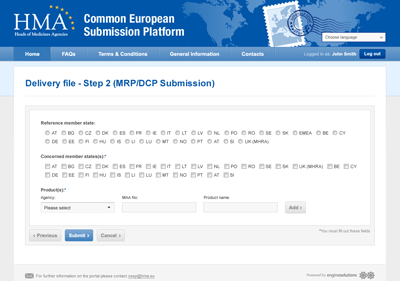
Solution
The CESP Project for Human and Veterinary Electronic Submissions provides a simple and secure mechanism for exchange of submission information between applicants and agencies, covering the following:
- National, Mutual Recognition Procedure (MRP) and Decentralised Procedure (DCP) application types
- Initial, variation and renewal submissions
The aim of the system is to:
- Provide a secure method of communicating with the regulatory agencies via one platform
- Allow submission of an application once to reach all required agencies
- Reduce the burden for both industry and regulators of submitting/handling applications on CD-Rom and DVD
Results
When submitting through CESP, the parallel sending of CDs or DVDs, including the paper submission letter and/or ‘application form’ to the national authority is no longer necessary. CESP is available for all EU Member States and authorization applicants and holders, for both human and veterinary medicinal products. CESP can be accessed via http://cesp.hma.eu.
Every company must appoint its own ‘company administrator’, who establishes and maintains the access to CESP for employees of that company.